
London
The King’s College London / Royal Free / UCL Gene Therapy Hub
The London Hub is made up of King’s College London, University College London and the Royal Free NHS Foundation Trust. The goal of the London Hub is to bring together leaders in gene therapy to provide GMP gene therapy vector manufacturing capabilities to academic partners across the UK. The focus will be on providing gene therapy products for early-stage clinical trials for rapid translation of laboratory findings into clinical evaluation. Central to the London Hub’s manufacturing capabilities is the Gene Therapy Vector Facility (GTVF) at King’s College London.
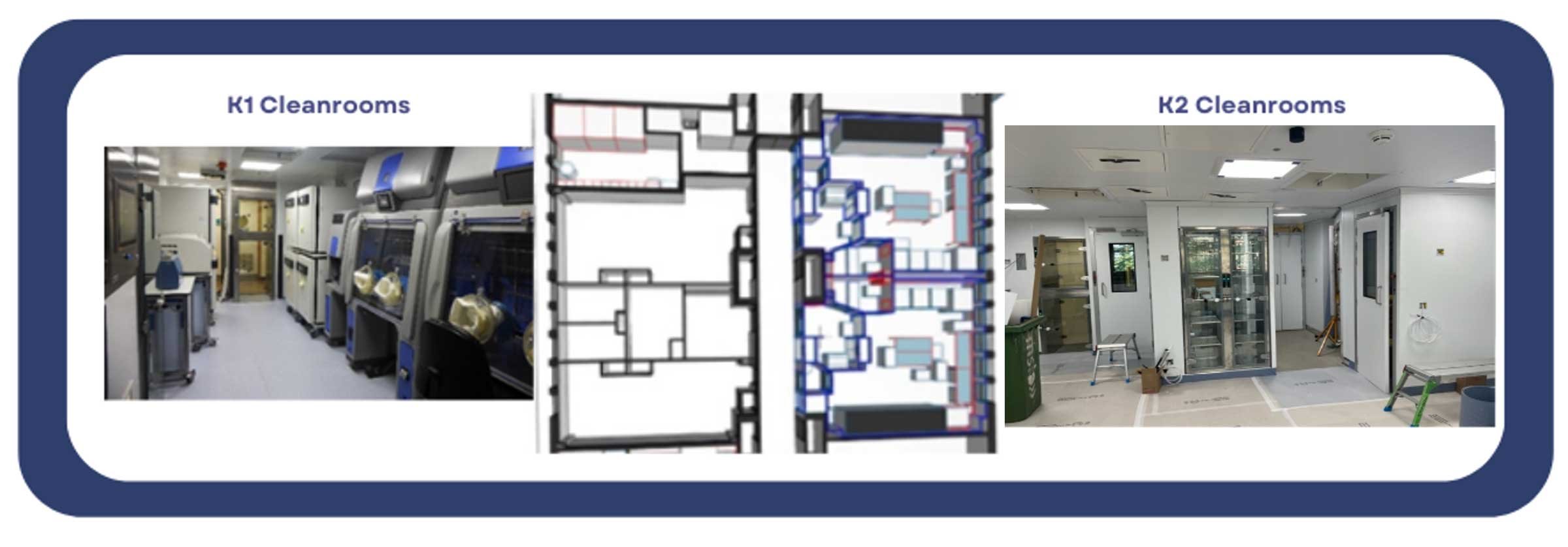
The GTVF brings an established facility to the London Hub, where over nearly 20 years, 100+ batches of GMP viral vector have been manufactured for early-stage clinical trials and an established academic and commercial community exists. The GTVF offers manufacture of GMP lentiviral vector (LV), retroviral vector (RV) and soon adeno-associated viral vector (AAV). The GTVF is expanding its GMP cleanroom capacity from 4 active cleanrooms to 8 cleanrooms over the next 12-24 months in order to deliver batches of GMP-grade vectors with minimised lead times. Below is an image of a schematic and associated images of a number of cleanrooms based in Denmark Hill in South London. The “K2” facility, which is currently under construction will expand LV and RV manufacturing capacity and is set to open in 2024.
The GTVF is set to expand its offering to include AAV vectors which are increasingly used for a wide range of gene therapy applications. The GTVF will initially focus its activities on AAV9 and the facility will have capacity to produce 4-5 AAV GMP batches per year for use in clinical trials requiring small doses of AAV. The dedicated AAV and Process Development teams at the GTVF have been established and are working to move manufacturing technology from adherent cell factory systems into adherent bioreactors, and then suspension bioreactors in order to enable larger batch sizes to be produced.
The manufacturing capabilities of the London Hub provides the academic community with an essential translational bridge into early phase clinical trials. With a combination of expertise, value and legacy, the GTVF is able to offer a partnership model, guiding academics through the process of viral vector manufacture for their clinical trials. Although many commercial CDMOs are able to manufacture viral vectors, the GTVF, as part of the London Hub, is focused on cost-effective manufacturing to support rapid translation of laboratory findings into clinical evaluation.
Please get in touch at for more information or to discuss your viral vector manufacturing needs.

Key People
London IHfGT Leadership Team:
- Hub's Director: Prof Robin Ali
- Chairman of KCL GTVF Board: Prof Simon Howell
- Hub's Operational Director: Kate Barlow
Supported by KCL GTVF Management:
- GTVF Operational Director: Dr Keith Meaney
- GTVF Head of Projects: Dr Joanna Przysowa
- GTVF Head of Business Development: Dr Henry Pegram
Work Package Leaders:
- Head of LV Production: Dr Sabine Domning (KCL)
- Head of AAV Delivery: Ferdos Hashi (KCL)
- Process Development Co-Lead: TBC
- Process Development Co-Lead: Prof Mark Lowdell (RF)
- Process Development Co-Lead: Prof Martin Pule (UCL)
- Director of Skills & Training: Prof Qasim Rafiq (UCL)
- Skills & Training Co-Lead: Dr Joanna Przysowa (KCL)